China Biotech Expansion Pushes Lab Monkey Prices to Record Highs
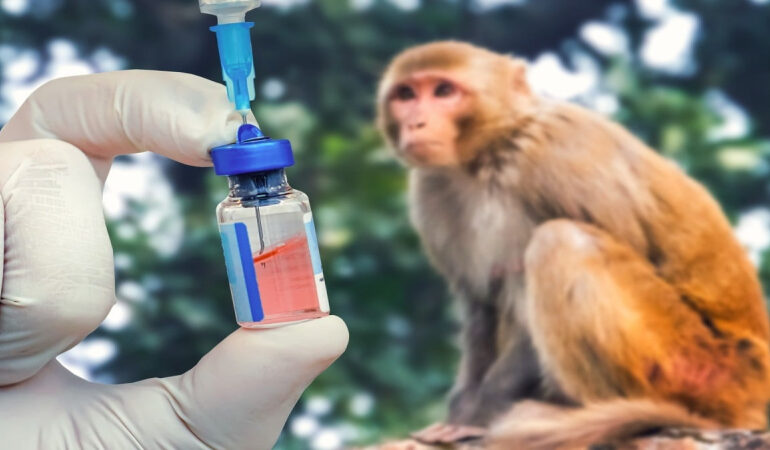
China’s accelerating biotechnology expansion is driving an unexpected cost surge in one of the industry’s most critical research resources, laboratory monkeys used in preclinical drug testing. Prices for experimental monkeys have climbed to around 140,000 yuan per animal, a level that now exceeds the country’s average annual wage and marks a sharp increase over the past five years.
Government procurement data shows that the cost of lab monkeys has roughly doubled during that period, reflecting tightening supply conditions as pharmaceutical research intensifies. These animals play a central role in early stage drug development, where researchers evaluate safety profiles, absorption rates, metabolic pathways, and toxicity before advancing candidates into human trials.
The spike in demand is closely linked to a revival in China’s biotech financing environment and a growing number of cross border drug licensing agreements. Chinese biotech firms have entered into a wave of partnerships with global pharmaceutical companies, out licensing innovative compounds or co developing new therapies. These transactions have injected fresh capital into research pipelines and pushed a record number of candidate drugs into preclinical evaluation.
Analysts estimate that domestic supply of experimental monkeys between 2025 and 2027 will range from roughly 49,000 to just over 52,000 animals annually. While this represents a stable breeding base, the rapid expansion of biotech research programs has tightened availability. Drug developers focused on oncology, autoimmune diseases, metabolic disorders, and neurological conditions are all competing for limited preclinical resources.
The imbalance between supply and demand has also increased lead times for research institutions seeking animal models. Smaller biotech start ups, which rely heavily on venture funding milestones, face particular pressure when delays in preclinical testing threaten development timelines. Larger pharmaceutical firms, with deeper capital reserves, are often better positioned to secure supply through long term agreements.
China’s biotech sector has undergone significant transformation in recent years, shifting from a generic drug manufacturing base to an innovation driven ecosystem. Regulatory reforms have accelerated clinical approvals and improved intellectual property protections, encouraging domestic companies to pursue original drug discovery. As more locally developed therapies advance toward global markets, demand for rigorous preclinical validation continues to grow.
The rising cost of laboratory monkeys also highlights broader structural challenges within biomedical supply chains. Breeding primates suitable for research requires strict compliance with animal welfare standards, disease control protocols, and specialized facilities. Expanding capacity is capital intensive and subject to regulatory oversight, limiting how quickly supply can respond to surging demand.
For investors and policymakers, the trend underscores how upstream research inputs can become bottlenecks in high growth sectors. As China positions itself as a global biotech innovation hub, managing resource constraints in preclinical testing will be essential to sustaining momentum in drug development and international partnerships.



